
Streamlined production of genetically modified T cells with activation,
transduction and expansion in closed-system G-Rex bioreactors
CHRISTINE GAGLIARDI, MARIAM KHALIL & AARON E. FOSTER
Bellicum Pharmaceuticals, Houston, Texas, USA
Abstract
Background: Gas Permeable Rapid Expansion (G-Rex) bioreactors have been shown to efficiently expand immune cells
intended for therapeutic use, but do not address the complexity of the viral transduction step required for many engineered
T-cell products. Here we demonstrate a novel method for transduction of activated T cells with Vectofusin-1 reagent. Trans-
duction is accomplished in suspension, in G-Rex bioreactors. The simplified transduction step is integrated into a stream-
lined process that uses a single bioreactor with limited operator intervention. Methods: Peripheral blood mononuclear cells
(PBMCs) from healthy donors were thawed, washed and activated with soluble anti-CD3 and anti-CD28 antibodies either
in cell culture bags or in G-Rex bioreactors. Cells were cultured in TexMACS GMP medium with interleukin (IL)-7 and
IL-15 and transduced with RetroNectin in bags or Vectorfusin-1 in the G-Rex. Total viable cell number, fold expansion, via-
bility, transduction efficiency, phenotype and function were compared between the two processes. Results: The simplified
process uses a single vessel from activation through harvest and achieves 56% transduction with 29-fold expansion in
11 days. The cells generated in the simplified process do not differ from cells produced in the conventional bag-based process
functionally or phenotypically. Discussion: This study demonstrates that T cells can be transduced in suspension. Further,
the conventional method of generating engineered T cells in bags for clinical use can be streamlined to a much simpler, less-
expensive process without compromising the quality or function of the cell product.
Key Words: autologous, bioreactors, cell therapy, chimeric antigen receptor T cells
Introduction
Manufacturing autologous T-cell therapies can be a
complex process. Gene transfer by viral transduction
to generate chimeric antigen receptor (CAR) T cells,
for example, has commonly been performed by
transferring activated T cells to bags or plates coated
with RetroNectin (Takara Bio USA, Inc., Mountain
View, CA, USA). The coating step requires dilution,
transfer and incubation of the RetroNectin solution
with subsequent washing and viral incubation before
T cells are added. After transduction, cell suspen-
sions are washed and transferred to additional cul-
ture vessels. Several of these steps are often open,
labor-intensive and cumbersome.
Alternatively, transduction of T cells in suspen-
sion could simplify viral gene transfer and reduce
time, cost and risk of errors. Vectofusin-1 (Miltenyi
Biotec Inc., Auburn, CA, USA) is a synthetic pep-
tide that can enhance viral transduction when added
in solution with virus and cells [1]. Vectofusin-1
forms a-helical nanofibrils that associate with viral
particles that enhance virus-cell interactions [2].
Transduction in solution with Vectofusin-1 elimi-
nates the need for surface coatings, expanding the
potential vessels in which T cells might be trans-
duced.
Gas Permeable Rapid Expansion (G-Rex; Wilson
Wolf Corporation, Saint Paul, MN, USA) bioreac-
tors are disposable single-use vessels specifically engi-
neered to culture and expand immune cells. Like
gas-permeable bags, G-Rex vessels are dependent on
incubation in standard cell culture incubators. G-
Rex technology relies on a silicone gas-permeable
membrane on which cells reside and through which
oxygen and carbon dioxide can be passively, effi-
ciently exchanged [3,4].
The G-Rex has been shown to be effective in
expanding tumor-infiltrating lymphocytes (TILs)
[5,6], natural killer (NK) cells [79],Vg9Vd2 T cells
[10], regulatory T cells [11] and virus-specific T cells
(VSTs) [1215]. In the context of genetically modi-
fied cells, such as CAR T cells, G-Rex bioreactors do
Corres ponde nce: Christine Gagliardi, PhD, Bellicum Pharmaceuticals, 2130 W. Holcombe Blvd Suite 800, Houston, Texas, USA. E-mail: [email protected]
(Received 18 April 2019; accepted 16 October 2019)
ISSN 1465-3249 Copyright © 2019 International Society for Cell and Gene Therapy. Published by Elsevier Inc. All rights reserved.
https://doi.org/10.1016/j.jcyt.2019.10.006
Cytotherapy, 2019; 21: 12461257
not directly address the added complexity of gene
transfer, however, viral transduction in solution with
Vectofusin-1 may allow for an integrated process in
which all unit operations are carried out in a single
G-Rex bioreactor.
The objective of this study was to simplify and
optimize the transduction and expansion steps of
manufacturing genetically modified T cells for clini-
cal use. We demonstrate a streamlined, novel
method for transducing T cells with retrovirus
directly in a G-Rex bioreactor using the Vectofusin-1
reagent, followed by robust expansion with no need
for operator intervention until harvest.
Materials and Methods
Starting material
Buffy coats from healthy donors were purchased
through the Gulf Coast Regional Blood Center
(Houston, TX, USA). Peripheral blood mononu-
clear cells (PBMCs) were isolated from buffy coat
using density gradient centrifugation with Lympho-
prep medium (STEMCELL Technologies Inc.,
Cambridge, MA, USA) per the manufacturer’s pro-
tocol. PBMCs were cryopreserved at 5 £ 10
7
cells/
mL in fetal bovine serum with 10% dimethyl sulfox-
ide and stored in liquid nitrogen until needed.
Culture imitation and T-cell activation
Cryopreserved PBMCs were thawed and washed in
cell culture medium. Small-scale experiments aimed
at understanding growth kinetics on the G-Rex
membrane were cultured and activated in a 50:50
mix of Gibco RPMI 1640 medium (Thermo Fisher
Waltham, MA, USA) and Clicks Medium EHAA
(FUJIFILM Irvine Scientific, Santa Ana, CA, USA)
with 10% fetal bovine serum. For transduction and
large-scale experiments, TexMACS GMP medium
(Miltenyi Biotec) was used.
Cells were counted and resuspended at
12 £ 10
6
cells/mL in medium with 15 ng/mL inter-
leukin (IL)-7, 5 ng/mL IL-15, 0.2 mg/mL anti-CD3
antibody and 0.5 mg/mL anti-CD28 antibody.
Cytokines and antibodies were from Miltenyi Biotec.
Cell suspension was transferred to VueLife “C” bags
(Saint-Gobain Cell Therapy, Gaithersburg, MD,
USA) and incubated at 37˚C, 5% CO
2
for 2 days.
For activation in G-Rex 6M 10 cm
2
plates (Wil-
son Wolf Corporation), PBMCs were thawed,
washed and counted. Aliquots of 1 £ 10
7
viable cells
were resuspended in 2.0, 4.0 or 10.0 mL medium
with 15 ng/mL IL-7, 5 ng/mL IL-15, 0.2 mg/mL
anti-CD3 antibody and 0.5 m g/mL anti-CD28 anti-
body. The cell suspensions were transferred to
individual 10-cm
2
wells and incubated at 37˚C, 5%
CO
2
for 2 days.
Transduction
Two high-titer retroviral constructs were used for
this study. One virus, used in the small-scale experi-
ments, carries a rapamycin-induced, caspase-9 safety
switch (iRC9) with a truncated CD19 marker that
was designed to work in combination with rimidu-
cid-controlled elements [16]. The other, used in the
large-scale process, carries a first generation prostate
stem cell antigen (PSCA) CAR with rimiducid-
inducible co-stimulation (inducible MyD88/CD40
[iMC]) and a CD34 epitope that can be used to mea-
sure transduction efficiency [1719].
For transduction in bags, 20 mg/mL RetroNectin
(Takara) in phosphate-buffered saline was incubated
in VueLife “AC” bags (Saint-Gobain) overnight at
4˚C. The RetroNectin solution was removed and
viral supernatant diluted in cell culture medium was
incubated in the bag for at least 30 min at room tem-
perature. Activated T cells were washed, resus-
pended in culture medium with 15 ng/mL IL-7 and
5 ng/mL IL-15 and added to the viral supernatant in
the bag. Bags were incubated at 37˚C, 5% CO
2
for 1
h, then flipped and incubated for 1824 h. After
incubation, cell suspensions were washed in cell cul-
ture medium, resuspended at 0.51.0 £ 10
6
cells/
mL in medium with 15 ng/mL IL-7 and 5 ng/mL IL-
15, transferred to VueLife “C” bags and incubated at
37˚C, 5% CO
2
.
For transduction in G-Rex bioreactors, Vectofu-
sin-1 (Miltenyi Biotec) was diluted to 1 mg/mL in
sterile water. Vectofusin-1 (10 mg/mL final concen-
tration) was combined with retroviral supernatant at
multiplicity of infection (MOI) 130. The solution
was added to activated T cells in the G-Rex and incu-
bated at 37˚C, 5% CO
2
for 1824 h. After incuba-
tion, cell culture medium with IL-7 and IL-15 was
added to the maximum volume of the vessel (10 mL/
cm
2
) and placed back in the incubator.
Expansion
For expansion in bags, samples were taken daily to
measure glucose and lactate concentrations, viable
cell density and viability. Cell suspensions were
diluted to 0.51.0 £ 10
6
cells/mL with fresh medium
when the cell density exceeded 2 £ 10
6
cells/mL.
For expansion in G-Rex bioreactors, samples
were taken daily to measure glucose and lactate con-
centrations. If sampled for count and viability, 90%
of medium was removed, cells were pipetted into sus-
pension, volume was measured and a sample was
Streamline production of genetically modified T cells 1247

taken. Fresh medium with cytokines was added to
the maximum volume.
In-process monitoring
Glucose concentration was measured with a CVS
Health Advanced Blood Glucose Meter (CVS
Health, Woonsocket, RI, USA). Lactate concentra-
tion was measured with the Lactate Plus Meter
(Nova Biomedical, Waltham, MA, USA). Cell count
and viability were measured on the Nucleocounter
NC-3000 (Chemometec, Allerod, Denmark).
Flow cytometry
Flow cytometry was performed on a Novocyte 3000
(ACEA Biosciences, Inc., San Diego, CA, USA) and
analyzed with ACEA NovoExpress software. Cells
were analyzed for transgene expression, CD4:CD8
ratio and memory population distribution. The follow-
ing antibodies were used in the flow panel: CD34-PE
(ABNova, Walnut, CA, USA) or CD19-PE for trans-
gene detection, CD4-PerCP, CD45RA-PE Cy7 (BD
Biosciences, San Jose, CA, USA), CD62L-APC,
CD3-Alexa Fluor 700 and CD8-BV510. Unless noted,
antibodies were from BioLegend. Zombie yellow fix-
able viability dye (BioLegend, San Diego, CA, USA)
was used to remove dead cells from the analysis. BD
Biosciences, San Jose, CA, USA.
Apoptosis assay
Cells from small-scale experiments were incubated
for 4 h with or without 10 nmol/L temsirolimus to
test function of the iCasp-9 transgene. After incuba-
tion, cells were stained with Annexin V-FITC,
CD19-PE, CD3-BV421 and zombie yellow fixable
viability dye in Annexin V binding buffer. All
reagents were from BioLegend. CD3
+
CD19
+
cells
were plotted on Annexin V against viability dye
graphs to show viable cells (Annexin V
/viability
dye
), apoptotic cells (Annexin V
+
/viability dye
)
and non-viable cells (Annexin V
+
/viability dye
+
).
Cytokine secretion assay
Cryopreserved aliquots of cells generated in the G-
Rex and bag large-scale processes were thawed,
washed and counted. Cells were resuspended at
2 £ 10
6
viable cells/mL in culture medium with IL-7
and IL-15 and incubated at 37˚C with 5% CO
2
over-
night. On the same day, 1 £ 10
4
PSCA
+
human pan-
creatic cancer cells (ATCC, Manassas, VA, USA)
were seeded per well of a 96-well plate and placed in
the incubator. The next day, T cells were harvested,
washed, counted and resuspended in medium at
2 £ 10
6
cells/mL. Then, 1 £ 10
4
T cells were plated
in each well of the 96-well plate containing the
PSCA
+
cells for an effector to target ratio of 1:1.
Each sample was tested with and without 10 nmol/L
rimiducid, which triggers the co-stimulation. After
48-h incubation, 25 mL supernatant was collected
for cytokine analysis with the Human Cytokine/Che-
mokine Magnetic Bead kit (Millipore) according to
the manufacturer’s instructions.
Closed-system process
For the good manufacturing practice (GMP)-com-
patible G-Rex (100 cm
2
) closed-system process,
PBMCs were thawed and activated in the G-
Rex100MCS in 40 mL (0.4 mL/cm
2
) on day 0. On
day 2, transduction reagents, including viral superna-
tant (MOI 5) and Vectofusin-1, were added. On day
3, medium was added to the maximum volume of
the vessel (1 L, 10 mL/cm
2
). Samples of the superna-
tant were taken daily for glucose and lactate concen-
tration measurement through the sampling port.
Cells were harvested on day 11 with the GatheRex
device (Wilson Wolf Corporation). A parallel bag-
based process was run for comparison. Briefly,
PBMCs from the same donors were thawed and acti-
vated in bags, followed by transduction at MOI 5 in
RetroNectin-coated bags on day 2. Cells were
washed and transferred to cell culture bags for
expansion. Samples were taken to measure viable
cell density and determine when cultures required
dilution. Total viable cells (TVCs), total transgene
Figure 1. Bag and G-Rexbased process overview.
1248 C. Gagliardi et al.

positive cells, percent transgenic cells, fold expan-
sion, phenotype and cytokine secretion were com-
pared between processes. Process flow charts are
shown in Figure 1.
Statistics
Statistics were calculated in GraphPad Prism. Version
7.03. Data are presented as mean § standard error of
the mean. Unpaired Student’s t-test was used to com-
pared differences between groups. One-way analysis
of variance was used compare multiple groups.
Results
Evaluation of T-cell expansion in G-Rex bioreactors
To observe kinetics of expansion in the G-Rex, 1 day
after bag transduction, T-cell cultures were washed,
resuspended in fresh medium and seeded in 10 cm
2
G-Rex 6-well plates at 0.031.00 £ 10
6
cells/cm
2
(i.e., 0.310.0 £ 10
6
cells/well). Then, 1 £ 10
7
cells
were cultured in a bag for comparison. Cells were
maintained in culture up to 3 weeks with count and
viability measurement and medium exchange every
34 days. In all conditions, cell proliferation pla-
teaued at 1014 days before expansion and viability
decreased (Figure 2). Lower seeding densities
allowed for higher fold expansion, whereas higher
densities resulted in more total viable cells. The max-
imum number of cells in one well (seeded at 1 £ 10
6
cells/cm
2
) peaked at 2.5 £ 10
8
total cells, or
2.5 £ 10
7
cells/cm
2
, which corresponds to 25-fold
expansion. Cells from the same donor, seeded a
lower density (0.06 £ 10
6
cells/cm
2
), expanded up to
42-fold to 2.6 £ 10
7
total cells. There was no differ-
ence it total cells at any timepoint between condi-
tions starting with 1 £ 10
7
in bags or G-Rex.
To determine the maximum density of the G-Rex
membrane, transduced T cells were plated at
0.52.0 £ 10
6
cells/cm
2
(i.e., 520 £ 10
6
cells/10
cm
2
well). G-Rex plates with maximum medium vol-
umes of 4 mL/cm
2
and 10 mL/cm
2
were tested to
determine if there is any benefit of the increased
medium height. Daily samples of supernatant were
taken for glucose monitoring, but the cell layers were
left undisturbed until harvest. To monitor prolifera-
tion, triplicate wells were harvested 3, 6 or 9 days
after seeding. Cytokines were added every 3 days to
replicate the environment in cell culture bags where
fresh medium is added as cell density is diluted. Cell
density at harvest was higher for cells cultured in the
6M format (10 mL/cm
2
medium; Figure 3). A
5 £ 10
5
cells/cm
2
seeding density resulted in harvest
density of 2.5 £ 10
7
cells/cm
2
(2.5 £ 10
8
TVCs on
10 cm
2
) in the smaller G-Rex compared with
4.2 £ 10
7
cells/cm
2
(4.2 £ 10
8
TVCs) in the 6M
G-Rex. There was little difference in harvest among
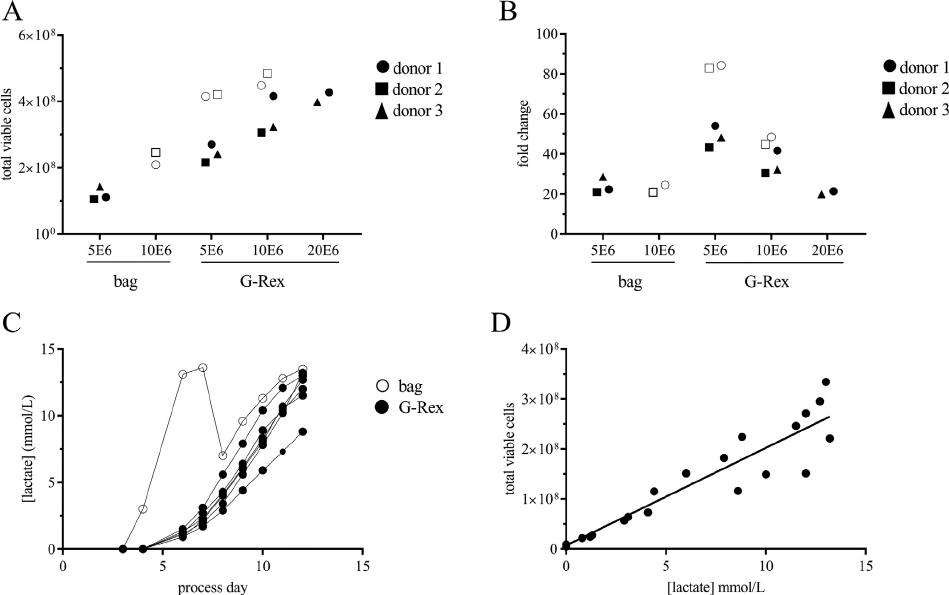
Figure 2. Kinetics of transduced T-cell proliferation, sampling to count every 34 days. Total viable cells (A, B) and fold expansion (C, D)
for 2 donors (A, C and B, D) grown in bags or 10 cm
2
G-Rex wells seeded with 0.310.0 £ 10
6
cells/well (0.03 1.0 £ 10
6
cells/cm
2
). Total
viable cells or fold change from day of seeding (y-axis) are plotted against process day (x-axis). Open circles represent data from bags; closed
circles represent data from G-Rex wells. Marker color is shaded according to seeding density. All conditions were resuspended and counted
every 34 days.
Streamline production of genetically modified T cells 1249
seeding 5 £ 10
5
or 1 £ 10
6
cells/cm
2
(42 § 0.3 £ 10
6
and 47 § 1.8 £ 10
6
TVCs) in the 6M plates and
2.0 £ 10
6
(41 § 1.5 £ 10
6
cells/cm
2
) in the smaller
plates (Figure 3).
To further optimize and simplify culture in the G-
Rex, the cytokine feeding schedule was evaluated.
The standard condition was supplementing cultures
with 15 ng/mL IL-7 and 5 ng/mL IL-15 every
3 days. Additional schedules were 45 ng/mL IL-7
and 15 ng/mL IL-15 given on the day of seeding
(i.e., 3x concentration once), 30 ng/mL IL-7 and
10 ng/mL IL-15 given on the day of seeding (i.e., 2x
concentration once) and 15 ng/mL IL-7 and
5 ng/mL IL-15 on the day of seeding and 5 days later
(i.e., 1x concentration twice in 9 days). There was no
difference in TVCs or viability in cultures given 3x
the usual amount of cytokines (45 ng/mL IL-7 and
15 ng/mL IL-15) on the day of seeding compared
with giving the standard concentration three times
over the course of expansion. Data from the same
experiments revealed a strong correlation between
lactate concentration in the G-Rex and the TVC
number (Figure 3).
Optimization of transduction in G-Rex bioreactors
PBMCs activated in bags were washed and transduced
with an MOI of 1 in 2.0, 4.0, or 10.0 mL in 10 cm
2
G-
Rex 6M plates (i.e., 0.2 mL/cm
2
,0.4mL/cm
2
or 1.0 mL/
cm
2
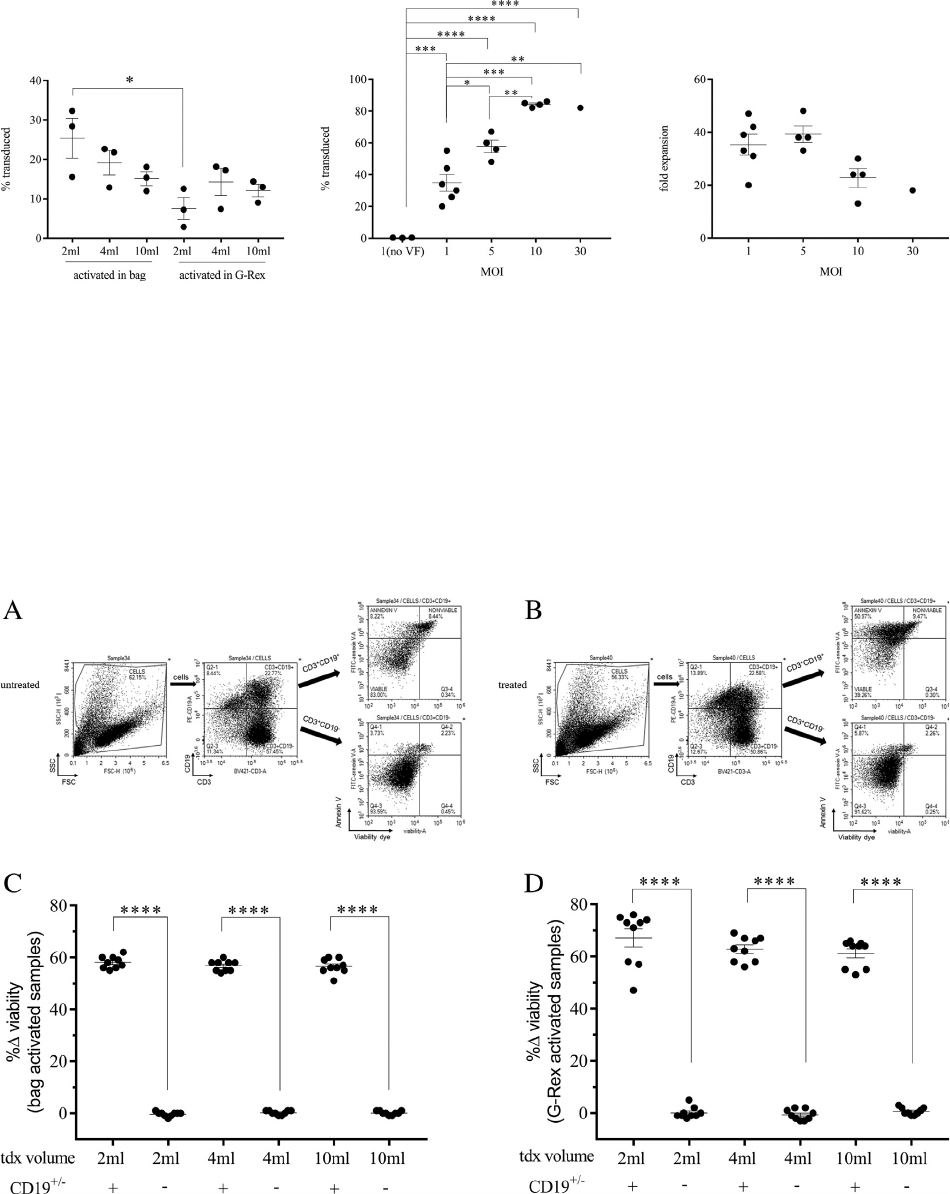
). Transductions were successful at all volumes, rang-
ing from 25 § 5% at 0.2 mL/cm
2
to 15 § 2% at 1.0 mL/
cm
2
(Figur e 4A). At 0.4 mL/cm
2
, transduction efficiency
increases with increased MOI. A MOI of 1, 5, 10 and 30
resulted in transduction efficiencies of 35 § 5%, 58 §
4%, 84 § 1% and 82%, respectively (Figure 4B). Expan-
sion of transduced T cells was negatively impacted by
high MOI (Figure 4C). The average fold expansion of
samples transduced with MOI of 1 and 5 was 37 § 3-
fold compared with 21 § 3-fold at MOIs of 10 and 30.
PBMCs were also activated directly in G-Rex
bioreactors before transduction. To maintain a low
volume for transduction without having to wash or
remove supernatant, cells were activated in 2.0, 4.0
or 10 mL in 10 cm
2
G-Rex 6M plates (i.e., 0.2 mL/
cm
2
, 0.4 mL/cm
2
or 1.0 mL/cm
2
). On the day of
transduction, Vectofusin-1 was mixed with retrovirus
(MOI 1), and then added to the activated cells.
Figure 3. G-Rex maximum supported cell density and lactate accumulation. Total viable cells (A) and fold change (B) of transduced T cells
expanded in bags or G-Rex starting with 5 £ 10
6
,1£ 10
7
or 2 £ 10
7
cells. Black markers represent experiments with 4 mL/cm
2
G-Rex
plates; white markers represent experiments with 10 mL/cm
2
G-Rex 6M plates. (C) Sample plot from 1 experiment of lactate concentration
(y-axis) plotted against process day (x-axis) for 7 individual culture conditions. Open markers represent bag culture; closed markers repre-
sent G-Rex 6M culture. (D) Total viable cells (y-axis) plotted against lactate concentration (y-axis). Data points collected from multiple
donors and experiments. Solid line represents linear regression calculated in GraphPad; R
2
= 0.9081.
1250 C. Gagliardi et al.
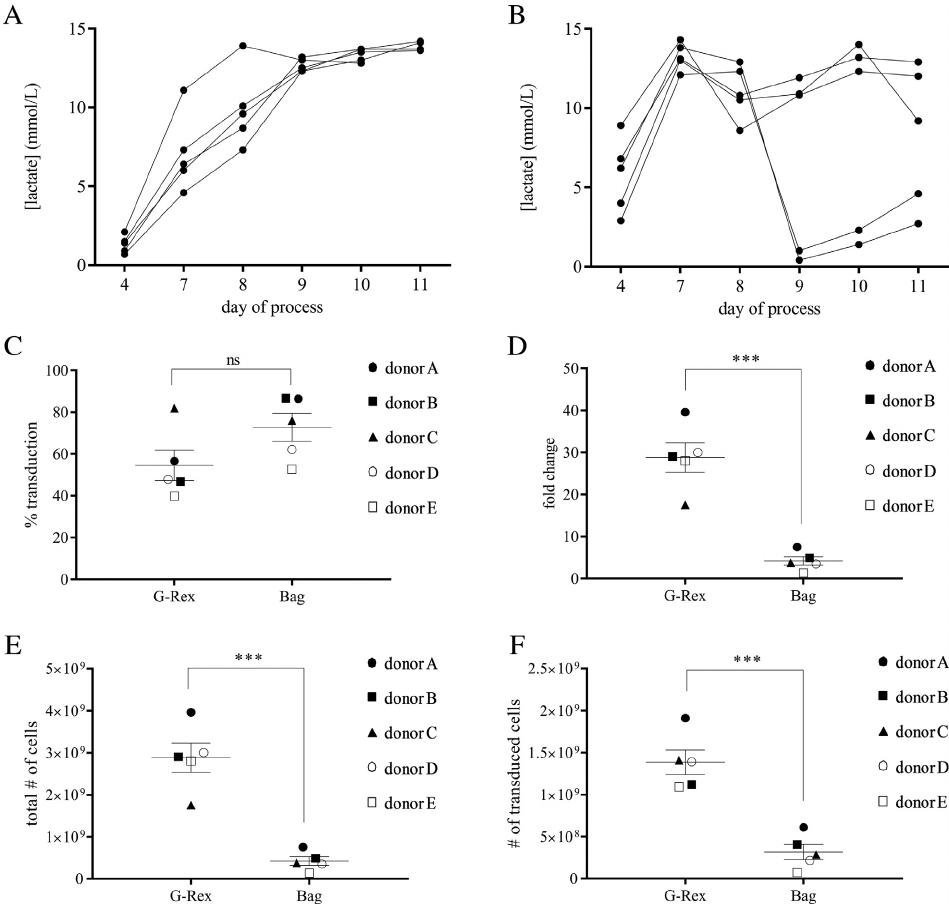
Overall, transduction efficiency was lower than com-
parable conditions transduced after bag activation
and culture wash (Figure 4A). Nonetheless, 14 §
3% transduction was achieved after activation in 0.4
mL/cm
2
. Transgenic cells, expressing an inducible
caspase-9, generated by both methods and
transduced in the G-Rex were functionally active
and responded as expected when incubated with
temsirolimus to induce apoptosis (Figure 5). Via-
bility of cells expressing the transgene was reduced
60% with treatment, whereas viability did not
change in the non-transduced population.
Figure 4. Evaluation of transduction volume and MOI in 10 cm
2
G-Rex. (A) Percentage of transduced cells plotted against mL medium/
cm
2
for cells activated in bags or G-Rex before transduction in G-Rex. (B) Percentage of transduced cells plotted against MOI for cells acti-
vated in bags and transduced in G-Rex. No VF represents conditions incubated with MOI 1, but without Vectofusin-1 reagent. (C) Fold
expansion plotted against MOI. *P 0.05, **P 0.01, ***P 0.001, ****P 0.0001.
Figure 5. Transgene function after transduction in 10 cm
2
G-Rex. Example flow plots from one sample of untreated cells (A) or cells incu-
bated for 4 h with drug to induce apoptosis (B). The inducible caspase transgene is detected with the CD19 marker. Change in viability for
transduced (CD19
+
) and non-transduced (CD19
) fractions plotted against treatment group for cells activated in bags (C) or in G-Rex (D)
and then transduced in the G-Rex. Change in viability is calculated by the formula (untreated viability treated viability)/untreated viabil-
ity. ****P 0.0001.
Streamline production of genetically modified T cells 1251
Closed-system G-Rexbased process
In-process monitoring of lactate buildup was used as
a surrogate marker of cell proliferation in the G-Rex.
There was a slow accumulation of lactate over time
in the G-Rex (Figure 6A) compared with rapid
increase in the bag with reduction after medium
addition (Figure 6B). Transduction efficiency in the
G-Rex 100MCS was 55 § 7%, compared with 73 §
7% in the RetroNectin-coated bag process
(Figure 6C). Significantly more total viable cells (2.8
§ 0.4 £ 10
9
vs 4.2 § 1.0 £ 10
8
) and transgenic cells
(1.4 § 0.1 £ 10
9
vs 3.2 § 0.9 £ 10
8
) were harvested
from the G-Rex compared with the bag process
(Figure 6E and 6F). There was no difference in the
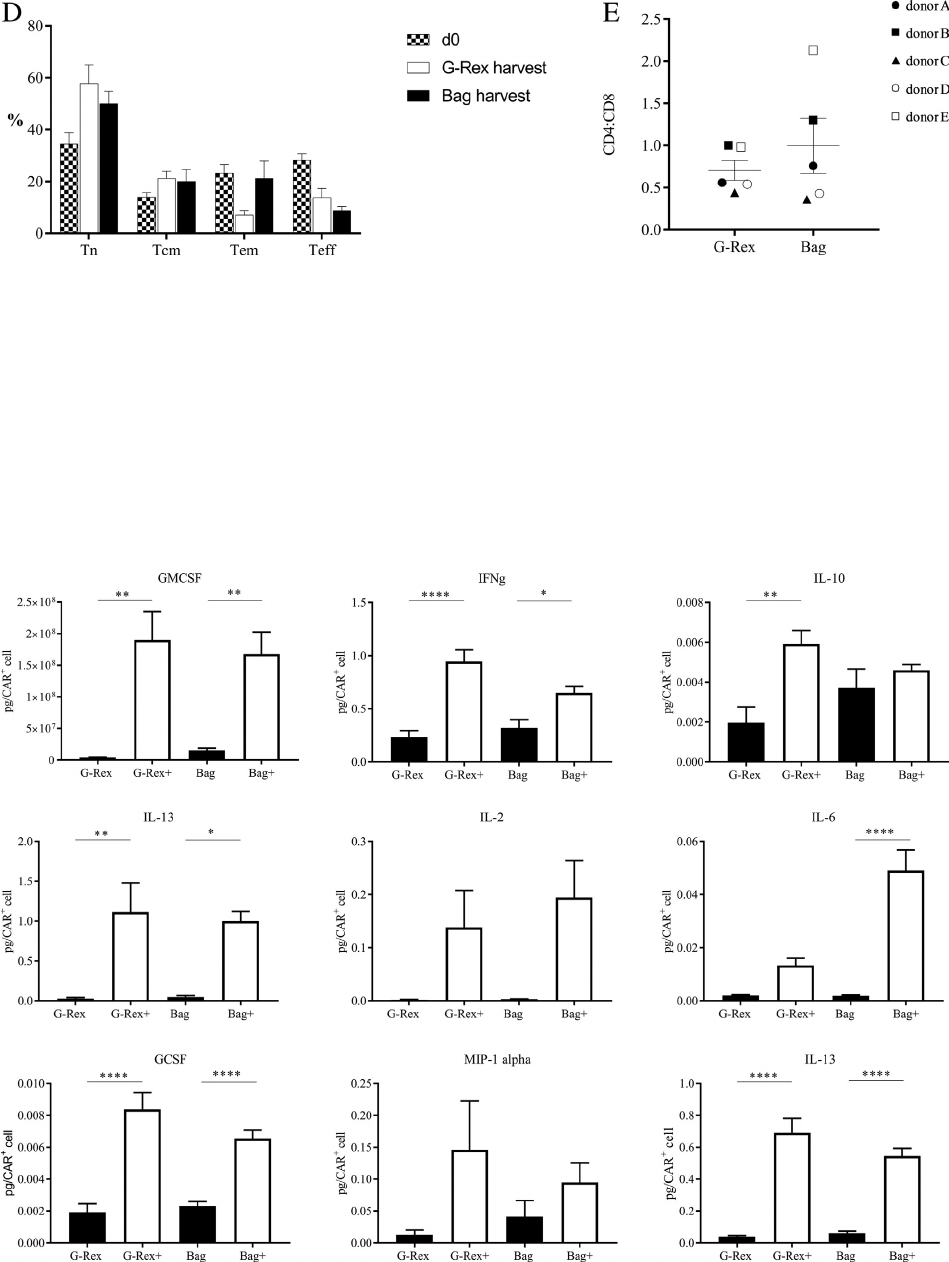
distribution of CD45RA and CD62L cell expression
or CD4:CD8 ratio between cells generated in the
two processes (Figure 7). Incubation of the G-
Rexcultured cells, transduced with a PSCA CAR
with inducible co-stimulation, on PSCA
+
target cells
resulted in a functional response measured by secre-
tion of cytokines (Figure 8) that did not differ from
that of cells produced in the conventional process.
The cost of reagents and consumables to generate
equivalent batch sizes from each process was esti-
mated based on currently available list prices
Figure 6. Large-scale G-Rex process compared with bag process lactate, transduction and expansion. (A, B) Lactate concentration (y-axis)
plotted against process day (x-axis) for large-scale G-Rex100MCS (A) and bag cultures (B). (CF) Comparison of transduction percentage
(A), fold change (B), total cells at harvest (C) and total transduced cells at harvest (D) of cells generated in the G-Rex and bag-based pro-
cesses. Each marker represents 1 donor. ***P 0.001, not significant P > 0.05.
1252 C. Gagliardi et al.
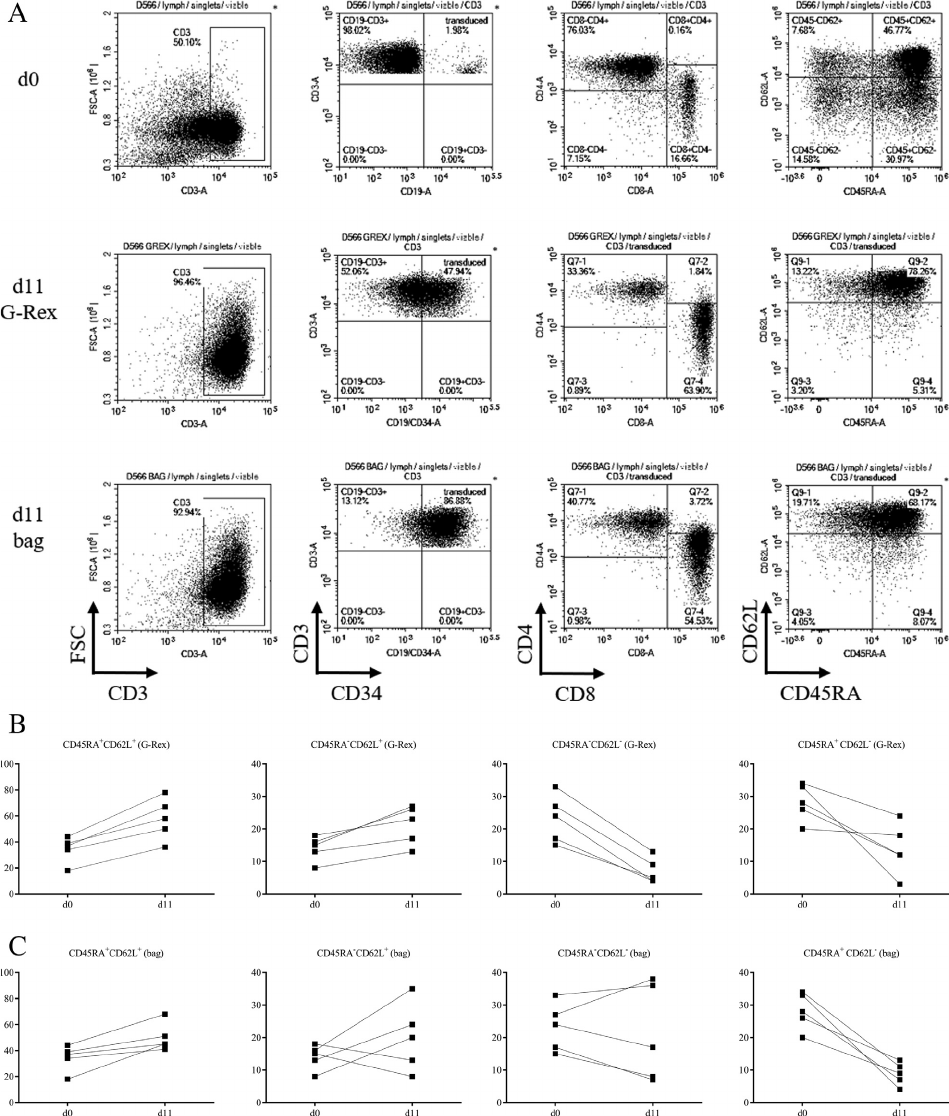
Figure 7. Phenotype of cells generated in the G-Rex and bag process is not different. (A) Sample flow plots for one donor on the initial day
of the process (d0), and after harvest from the G-Rex and bag. The transgene is detected using a CD34 marker. (B, C) Distribution of
CD45RA and CD62L expression on d0 and d11 for the G-Rex (B) and bag (C) processes. (D) Percentage of CD45RA
+
CD62L
+
(Tn),
CD45RA
CD62l
+
(Tcm), CD45RA
CD62L
(Teff) and CD45RA
CD62L
(Tem) from day 0 (checkered bar), G-Rex harvest (white
bar) and bag harvest (black bar). Two-tailed, unpaired t-test was used to compare G-Rex harvest to bag harvest, with no significant differ-
ence between the 2 for any population. (E) CD4:CD8 ratio at harvest from G-Rex or bags.
Streamline production of genetically modified T cells 1253
(Table 1). According to these calculations, materials
for the newly developed process would cost approxi-
mately 38% less than that for a conventional process.
Most of the cost reduction is due to the efficiency of
cell expansion in the G-Rex, which reduces the start-
ing cell number by 4-fold. A comparison between the
time required per operation for each process was also
made (Table 2), demonstrating a significant reduc-
tion of hands-on time for the G-Rexbased process.
Discussion
A major obstacle to manufacturing engineered
T cells is introduction of transge nes such as
CARs. Here we d emonstrate a simple method for
transducing T cells in suspens ion and show inte-
gration of the novel transduction s tep into a
streamlined manufacturing process that relies on a
single G-Rex bioreactor throughout. Al though
Figure 7 Continued.
Figure 8. Similar function of transgene in cells generated in the G-Rex and bag processes. Cytokine secretion, normalized to pg/CAR
+
cell,
of CAR T cells produced in the G-Rex and bag large-scale process after incubation with target cells § drug to provide co-stimulation. Black
bars represent conditions without drug; white bars represent conditions with drug. *P 0.05, **P 0.01, ***P 0.001, ****P 0.0001.
1254 C. Gagliardi et al.

functionally closed and efficient for expanding
cells [315], the G-Rex bioreactors have not, to
date, addressed the practical challenges of genetic
modification of T cells. The first goal of this study
was, the refore, to develop a transduction protocol
that would work for transducing T cells in suspen-
sion in the G-Rex.
Transduction of T cells has typically relied upon
RetroNectin-coated cell culture bags or plates. Given
that RetroNectin coating of the silicone membrane is
not possible, Vectofusin-1 reagent, which is added in
solution, was tested as an alternative for transduction
in the G-Rex. Incubation of retrovirus with activated
T cells in low volumes to increase cell/virus interac-
tion, but without transduction-enhancing reagents,
resulted in transduction efficiency of <1%. Adding
Vectofusin-1 increased transduction efficiency to
825% at MOI 1. Transduction efficiency was
increased with increasing MOI, and that increase
reached a maximum at MOI 10 (>80%). Medium
addition after transduction, without washing,
allowed for expansion of the transduced T cells in
the G-Rex, although expansion tended to be nega-
tively impacted at MOI 10.
Transduction and expansion of cells in the G-Rex
eliminate the complexity of transduction with Retro-
Nectin, but still require initial activation, with a wash
and transfer step. To further simplify the process,
activation in the G-Rex prior to transduction was
also evaluated. PBMCs activated in the G-Rex in 0.4
Table 1. Bag and G-Rex process cost comparison.
Bag-based process G-Rexbased process
Item Manufacturer Catalog no. Size List price Required
per run
Cost
per run
Required
per run
Cost
per run
TexMACS GMP
medium
Miltenyi Biotec 170-075-306 2 L $ 305.00 1.00 $ 305.00 1.00 $ 305.00
MACS GMP
Vectofusin-1
Miltenyi Biotec 170-076-165 1 mg $ 875.00 0.00 $ - 1.00 $ 875.00
G-Rex 100MCS Wilson Wolf Corp 81100-CS 3 pack $ 729.84 0.00 $ - 0.33 $ 243.28
MACS GMP CD3
pure
Miltenyi Biotec 170-076-116 1 mg $ 1750.00 0.58 $ 1015.00 0.08 $ 140.00
MACS GMP CD28
pure
Miltenyi Biotec 170-076-117 0.5 mg $ 895.00 1.45 $ 1297.75 0.20 $ 179.00
hIL-7, premium
grade
Miltenyi Biotec 130-095-363 100 mg $ 1150.00 0.13 $ 149.50 0.45 $ 517.50
hIL-15, premium
grade
Miltenyi Biotec 130-095-765 100 mg $ 1150.00 0.04 $ 46.00 0.15 $ 172.50
Retronectin, GMP
grade
Takara T202 2.5 mL $ 1442.00 0.50 $ 721.00 0.00 $ -
Cell culture bags Saint Gobain 290-C 10 pack $ 993.51 0.30 $ 298.05 0.00 $ -
Transduction bag Saint Gobain 290-AC 10 pack $ 993.51 0.10 $ 99.35 0.00 $ -
$3931.65 $2432.28
The cost of generating 1.4 £ 10
9
transduced cells in the bag- and G-Rexbased process. Cost for the bag process was calculated based on
scaling up to a process that could generate as many cells as the G-Rexbased process.
Table 2. Bag and G-Rex process time comparison.
Operation Bag-based process G-Rex-based process
Method Time (h) Method Time (h)
Activation Anti-CD3/CD28 in solution 1.00 Anti-CD3/CD28 in solution 1.00
Transduction Culture wash, transfer to
Retronectin-coated bags
4.00 Vectofusin-1 in solution 0.50
Transduction stop Culture wash, transfer bag 2.00 Medium addition 0.50
Expansion Feed and transfer, as needed 4.00 NA 0.00
Harvest Centrifuge 2.00 GatheRex 0.50
In-process monitoring Mix cell suspension, sample
for count (x6 d)
3.00 Sample supernatant for lactate (x6 d) 1.50
16.00 4.00
The estimated time, in hours, required for each operation in the bag- and G-Rexbased process. Estimates are based on a single operator
working in a non-GMP setting.
NA, not applicable.
Streamline production of genetically modified T cells 1255
mL/cm
2
were transduced with 15% efficiency. This
is slightly lower than cells activated in bags, but also
significantly reduces operator time and eliminates
wash and transfer of cells between activation and
transduction, reducing risk of contamination or other
errors.
These data demonstrate that the transduction step
can be streamlined to simple addition of reagents to
cells rather than RetroNectin coating, washing and
transferring cells pre- and post-transduction. This
method has the potential to reduce clean room time,
operator input and risk of contamination.
Using data from the small-scale experiments,
suggesting an optimal seeding density of
0.51.0 £ 10
6
cells/cm
2
andanactivationvolume
of 0.4 mL/cm
2
, a GMP-compatible process was
devised. Prioritizin g simpl icity over other parame-
ters, PBMCs were activated, transduced and
expanded in a single G-Rex bioreactor. Five runs
each of the simplified G-Rexbased process and
the standard bag process were performed in parallel.
There was no differen ce in phenotype or funct ion of
the cells generated in the two processe s. There was
a trend toward higher transduc tion in the bag,
although that difference was not statistically signifi-
cant. Expansion i n the G-Rex bioreactor resulted in
significantly more total vi able cells and transgenic
cells at harvest. The average harvest from the G-
Rex process was 1.4 § 0.1 £ 10
9
transgenic cells. A
hypothetical 80-kg patient who needs a dose of
5.0 £ 10
6
cells/kg could receive >3 doses from one
manufacturing process with the G-Rex. In compari-
son, a bag process, starting with t he same number
of cells, results in barely enough transgenic cells for
a single dose of the same size. Further, the G-
Rexbased process reduced the cost of mate rials by
38% and hand-on time by 75% to generate a batch
of 1.4 £ 10
9
transgenic cells.
This study demonstrates that T cells can be trans-
duced with retroviral vectors in solution in the G-Rex
bioreactor with addition of Vectofusin-1 as a trans-
duction enhancer. The simplified transduction step
allow for an entire process, from activation to har-
vest, to be carried out in a single vessel. Clinically rel-
evant levels of transgene expression and cell numbers
can be achieved by combining reagents in the G-
Rex, without complicated time-consuming coating
steps of traditional transduction.
The streamlined procedure reduces the hands-on
time of transduction to minutes rather than hours
and eliminates all cell transfer and wash steps. Fur-
ther, the increased output per starting material
reduces cost of materials compared with the standard
method. Cells can be expanded in the G-Rex with
limited operator intervention and without specialized
equipment.
Declaration of Competing Interest
All authors are employees of Bellicum Pharmaceuti-
cals. This research did not receive any specific grant
from funding agencies in the public, commercial or
not-for-profit sectors.
Author Contributions
Conception and design of the study: CG. Acquisition
of data: MK, CG. Analysis and interpretation of data:
MK, CG. Drafting or revising the manuscript: MK,
CG, AEF. All authors have approved the final article.
References
[1] Fenard D, Ingrao D, Seye A, Buisset J, Genries S, Martin S,
et al. Vectofusin-1, a new viral entry enhancer, strongly pro-
motes lentiviral transduction of human hematopoietic stem
cells. Mol Ther Nucleic Acids 2013;2:e90.
[2] Vermeer LS, Hamon L, Schirer A, Schoup M, Cosette J,
Majdoul S, et al. Vectofusin-1, a potent peptidic enhancer of
viral gene transfer forms pH-dependent alpha-helical nanofi-
brils, concentrating viral particles. Acta Biomater 2017;
64:259–68.
[3] Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H,
et al. Accelerated production of antigen-specific T cells for
preclinical and clinical applications using gas-permeable
rapid expansion cultureware (G-Rex). J Immunother 2010;
33(3):305–15.
[4] Bajgain P, Mucharla R, Wilson J, Welch D, Anurathapan U,
Liang B, et al. Optimizing the production of suspension cells
using the G-Rex “M” series. Mol Ther Methods Clin Dev
2014;1:14015.
[5] Forget MA, Haymaker C, Dennison JB, Toth C, Maiti S,
Fulbright OJ, et al. The beneficial effects of a gas-permeable
flask for expansion of tumor-infiltrating lymphocytes as
reflected in their mitochondrial function and respiration
capacity. Oncoimmunology 2016;5(2):e1057386.
[6] Jin J, Sabatino M, Somerville R, Wilson JR, Dudley ME,
Stroncek DF, et al. Simplified method of the growth of
human tumor infiltrating lymphocytes in gas-permeable
flasks to numbers needed for patient treatment. J Immun-
other 2012;35(3):283–92.
[7] Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J,
et al. Large-scale ex vivo expansion and characterization of
natural killer cells for clinical applications. Cytotherapy
2012;14(9):1131–43.
[8] Lapteva N, Szmania SM, van Rhee F, Rooney CM. Clinical
grade purification and expansion of natural killer cells. Crit
Rev Oncog 2014;19(1-2):121–32.
[9] Lapteva N, Parihar R, Rollins LA, Gee AP, Rooney CM.
Large-scale culture and genetic modification of human natu-
ral killer cells for cellular therapy. Methods Mol Biol 2016;
1441:195–202.
[10] Xiao L, Chen C, Li Z, Zhu S, Tay JC, Zhang X, et al. Large-
scale expansion of Vgamma9Vdelta2 T cells with engineered
K562 feeder cells in G-Rex vessels and their use as chimeric
antigen receptor-modified effector cells. Cytotherapy 2018;
20(3):420–35.
[11] Chakraborty R, Mahendravada A, Perna SK, Rooney CM,
Heslop HE, Vera JF, et al. Robust and cost effective expan-
sion of human regulatory T cells highly functional in a
1256 C. Gagliardi et al.
xenograft model of graft-versus-host disease. Haematologica
2013;98(4):533–7.
[12] Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM,
Craddock JA, Liu H, et al. Safety and clinical efficacy of rapidly-
generated trivirus-directed T cells as treatment for adenovirus,
EBV, and CMV infections after allogeneic hematopoietic stem
cell transplant. Mol Ther 2013;21(11):2113–21.
[13] Horlock C, Skulte A, Mitra A, Stansfield A, Bhandari S, Ip
W, et al. Manufacture of GMP-compliant functional adeno-
virus-specific T-cell therapy for treatment of post-transplant
infectious complications. Cytotherapy 2016;18(9):1209–18.
[14] Gerdemann U, Vera JF, Rooney CM, Leen AM. Generation
of multivirus-specific T cells to prevent/treat viral infections
after allogeneic hematopoietic stem cell transplant. J Vis Exp
2011(51):2736.
[15] Lapteva N, Vera JF. Optimization manufacture of virus- and
tumor-specific T cells. Stem Cells Int 2011(2011):434392.
[16] Duong MT, Collinson-Pautz MR, Morschl E, Lu A, Szy-
manski SP, Zhang M, et al. Two-dimensional regulation of
CAR-T cell therapy with orthogonal switches. Mol Ther
Oncolytics 2019;12:124–37.
[17] Philip B, Kokalaki E, Mekkaoui L, Thomas S, Straathof K,
Flutter B, et al. A highly compact epitope-based marker/sui-
cide gene for easier and safer T-cell therapy. Blood 2014;124
(8):1277–87.
[18] Foster AE, Mahendravada A, Shinners NP, Chang WC, Cri-
sostomo J, Lu A, et al. Regulated expansion and survival of
chimeric antigen receptor-modified T cells using small mole-
cule-dependent inducible MyD88/CD40. Mol Ther 2017;25
(9):2176–88.
[19] Narayanan P, Lapteva N, Seethammagari M, Levitt JM, Slawin
KM, Spencer DM. A composite MyD88/CD40 switch synergis-
tically activates mouse and human dendritic cells for enhanced
antitumor efficacy. J Clin Invest 2011;121(4):1524–34.
Streamline production of genetically modified T cells 1257
